SDSU Psychology Department
Experiment Management Tutorial for
Researchers/PIs
Revision 3
Introduction
The Sona Experiment Management System is used for the scheduling and management of human subject pools and the studies they participate in. Participants, researchers, principal investigators, and instructors all use the system for their respective purposes. As a researcher, you can set up your studies in the system, schedule sessions (timeslots), and grant or revoke credit after the session. All of this is handled through a web-based interface that you can access at any time, from any web browser. The site is located at http://sdsu.sona-systems.com/
The system has been configured by the Site Administrator, as per department policy related to research participation. It should be noted that the documentation herein may refer to features that are not enabled on your system. Contact the Site Administrator, whose contact information appears at the bottom of every page on the system, for more information.
System Basics
In the system, you create studies. Each study may have a number of timeslots, which are the times when you plan to run the study. Participants sign up for the timeslots by viewing a list of studies and available timeslots. You grant or revoke credit to participants after the session occurs.
Principal Investigator Special Note
This documentation applies to both researchers and principal investigators (PIs), when PI support is enabled by the administrator. A PI can perform all the same functions on a study as a researcher. This allows a PI to operate in an oversight role and monitor the progress of their studies, and step in on behalf of the researcher when necessary. Because the privileges are the same, throughout this documentation, the term “researcher” can be used interchangeably with “principal investigator” except where otherwise noted.
Participant ID Codes Special Note
If enabled by the administrator, the system will identify participants to you only by a unique, system-assigned ID code, and not by their name or email address, for privacy reasons.
Getting Started
The system works best if you use a web browser that is less than 2 years old. It works well with Internet Explorer version 4 and above, Netscape version 4 and above, and Firefox version 1.0 and above. It will work with other web browsers, and with older versions of Internet Explorer and Netscape, however the layout may not be as clean. No functionality will be lost by using an older web browser. This documentation assumes you have a basic knowledge of how to use the web. On this system, it is not necessary to use the Back button. You can always use the toolbar on the top to navigate to anywhere on the site.
Logging In
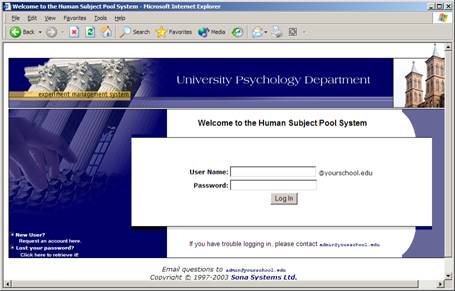
Your Site Administrator will provide you with a username and password to login to the site after your study has been approved. When you go to the front page of the site (the login page), you may see a link to request an account. This form is only for participants. Do not use this form to request a researcher account, as participant accounts have an entirely different set of privileges, and the privileges are not appropriate for a researcher.
Figure 1 – Login Page
Once you login, you may be asked to review and acknowledge your organization’s human subject policy. If required by the administrator, you will need to acknowledge this once every 6 months. You will see the Main Menu after you acknowledge the policy.
Figure 2 – Main Menu
Your login (also known as a session) will expire after a certain period of inactivity, usually 20-60 minutes. This is done for security purposes. If this happens, you can always log in again. When you are finished using the system, it is better to explicitly log out, to prevent any problems that may arise if someone uses your computer before the session expires.
Retrieving a Lost Password
If you have forgotten or do not have your password, you can ask the system to email your password to you. This option is located on the lower left corner of the main login page. After you submit the form the password will be emailed and should arrive in your inbox momentarily. If you provided an alternate email address (see the Email Address Options section of this documentation), it will be sent there. Otherwise, it will be sent to your main email address, which is derived from your user ID.
Logging Out
When you are finished using the system, choose Logout from the top toolbar to log out. You are now logged out. It is always a good security practice to close all your web browser windows as well, especially if you are using a computer that is shared by others.
Changing Your Password and Other Information
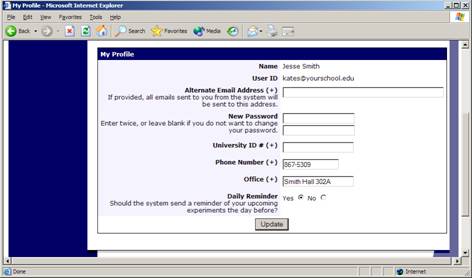
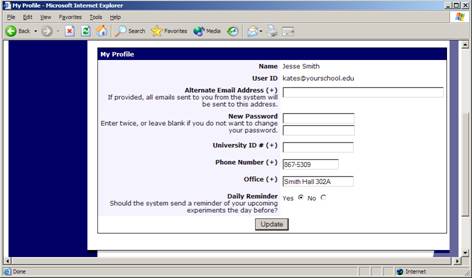
If you would like to change your password or other information about yourself, choose My Profile from the top toolbar. If you would like to change your password, type your new password (twice, for confirmation) in the boxes provided.
Figure 3 – Updating Your Profile
You will need to provide a phone number and office location so that research participants can contact you if they have questions related to your study. This contact information will be displayed to participants when they view information about the study. If you are a principal investigator, only your name will show (since the researcher is the primary point of contact for a study).
You may choose to receive a daily reminder (by email) with information about all of your study sessions scheduled for the following day.
Email Address Options
There are certain events in the system which will cause an email notification to be sent to you. Most often, these are notifications that a participant has signed up or cancelled their sign-up for your studies, but there are a few other cases where it may be used as well. The email address is also displayed to the participant when they view information about the study, in case they need to contact you with questions.
When you update your personal information, you will see a box where you may provide an alternate email address. If you provide such an address (this could be a Hotmail account, for instance), this is the address where any notifications will be sent, and this is also the address that will be displayed to other users (including participants in your studies).
Working with Studies
Most of your time on the system will be spent using the study-related features of the system. Be sure to read this section closely, in its entirety, as there are special features and situations you should be aware of.
Web-Based (Online) Studies
You may set up studies that are web-based (online), either within the Sona system (as a survey) or by using a link outside the system.
There are a few things to note about web-based studies:
- Once you indicate to the system that the study is web-based, you may not be able to change it so it is no longer web-based (but you can disable or delete the study). So, make this choice carefully
- Web-based studies are typically setup so there is one timeslot, and that timeslot contains the maximum number of participants you would like to participate, and the last date and time when they can participate (often, this is the end of the term). It is not recommended that you set up multiple timeslots for a web-based studies (it confuses participants), though the system will support it.
- It is generally assumed that participants will participate in an online study shortly after they sign up. Because of this, the system will expect you to grant credit to them soon after they sign up. If you are creating an online survey within the system, credit can be granted automatically, immediately after the participant completes the survey.
Throughout the sign-up process, participants are notified that the study is web-based. If the study is not administered by the system, then participants are not given the URL for the website until they have signed up, to ensure they do complete a sign-up in the system for the study. This restriction applies only to participants, and only to web-based studies administered outside the system.
For web-based studies administered outside the system, you will want to develop some method of linking the participant’s sign-up in the system to your online study, so you know who to grant credit to. To make this easier, the system has a built-in facility for this. If you enter the text %SURVEY_CODE% anywhere in the Study URL field for the study, the system will automatically replace this with a unique, numeric ID code when the URL is displayed to participants. If a participant is not viewing the URL (for example, the researcher is viewing the URL), this text will simply be removed. You can then program your online study to process and record this numeric ID, which will also be displayed on the pages in the system where you grant credit for participation. Note this special text must be in all capital letters, and surrounded by percent signs. You may confirm it was entered correctly because a sample URL will be displayed when you go to view (not edit) the study, below the normal study website link.
Online survey studies (surveys administered within the Sona system) are discussed in great detail in the section Online Survey Studies, later in this document. Please read it carefully before setting up an online survey study.
Studies for Pay
You may have a situation where participants are compensated for their participation in the study. They may or may not also receive credit for the study. If the study is not for credit, you may set it up as a pay-only study and specify the compensation amount. If participants are compensated and they receive credit, you should set it up as a credit study and indicate additional compensation in the study’s information section.
Regardless of the type of study, after a participant participates in a study (including studies that are for pay only), you should still go into the system and indicate their participation by granting the “credit” (or revoking it if they did not show). This allows the system to properly enforce certain restrictions on the participant and their studies.
Two-Part Studies
You may create a two-part study in the system. Often, these are studies involving memory research, where the participant must return a specified number of days after the first session. When creating a study, you may specify the day range for the second part of the study (e.g. 7 to 10 days after the first part). Participants are required to sign up for both sessions at the same time, to reduce the chance they will forget to sign up for the second part. Each part of a two-part study may have a different credit value and duration, but each part must be the same type – either both parts are for credit or both parts are for compensation.
You may specify that the second part of the study must be scheduled to take place at the exact same time as the first part (on a different date), or at any time on the dates that are the specified number of days after the first part.
You should ensure there are enough available timeslots for both parts of the study, or participants will be prevented from signing up for either part. Participants may cancel either part of their sign-up if necessary. If they cancel the first part, the second part is automatically cancelled as well. If they cancel only the second part and the first part has already occurred, and they would like to participate in the second part later, you will need to manually sign them up for the second part (if you are allowed to do so), or ask the administrator to handle this.
If you issue a no-show penalty for the first part of a two-part study, the second part of that participant’s sign-up will not be cancelled automatically, but you will be reminded of the situation in case you would like to cancel the second part. The cancellation is not automatic as there are some situations where automatic cancellation is not desirable.
Adding a Study
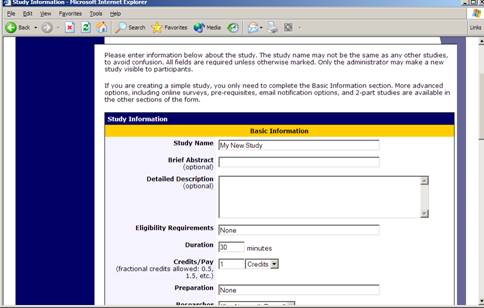
Figure 4 – Adding a New Study
To add a study, choose the Add New Study option from the top toolbar. You will need to pick from four possible types of studies. Please choose this carefully as you are not able to change this later.
After you choose, the study type you’ll see a form asking for more information. You will need to fill out a number of fields, which are explained in the following table. Some of the fields listed below may not appear, depending on how your system is configured and the type of study you selected. All fields must be filled out unless otherwise noted.
| Field | Explanation |
| Study Name | This is the experiment number you were assigned by the Site Administrator when your study was approved. |
| Brief Abstract | This field must be left blank, even though the system indicates that it is optional. |
| Detailed Description | This section should begin with the title for your study followed by a detailed description about the study. This section will be visible to participants when they click on the experiment number, and should be consistent with the title and description from your approved participant pool request. You may include basic HTML in this area, but please be sure you know what you are doing. If you would like to add a carriage-return (paragraph break), simply type in “<p>” (without the quotes). |
| Eligibility Requirements | If there are any restrictions on who may participate (for instance, only those who are left-handed), list them here. Otherwise, leave the field as-is. If you list any restrictions, these will be displayed on the list of studies, when participants view a list of all available studies. Note the system does not enforce these restrictions, but it is expected a participant will only sign up for a study in which they are qualified, since they would otherwise fail to receive credit. In most cases, you will leave this field as-is and set pretest participation restrictions, which you can do after you add the study. |
| Pre-Requisites (this feature might be disabled on your system) | If there are studies a participant must participate in before participating in your study, choose them here. You may select multiple studies, and on most systems, you hold down the Ctrl key and click the desired studies. |
You may specify that participants must have participated in all of the studies you specify, or at least one of the studies specified.
The system will handle enforcement of the pre-requisites in a strict or lenient fashion depending on how your system is configured. In strict enforcement mode, the participant must have received credit for the pre-requisite studies. In lenient enforcement mode, the participant must only be scheduled to participate in the pre-requisite studies (it is assumed they will go on to complete the pre-requisite studies). You can ask your subject pool administrator how this is configured, if it is of concern. If your system is in lenient enforcement mode, and a participant cancels a necessary pre-requisite for you study (they are warned of this situation), and you have configured your study so that the researcher will receive notifications of cancellations or sign-ups, then the researcher will receive notification of the pre-requisite problem and can contact the participant if necessary. Disqualifiers (this feature might be disabled on your system)If there are any studies a participant must not have participated in, please select them here. You may select multiple studies. The system will handle enforcements of the restriction, during the sign-up process. Course Restrictions If you would only like participants enrolled in certain courses to participate in your study, select the eligible courses here. Participants who are not in at least one of the courses you selected will not set the study when they go to view the list of available studies. You may choose No Restrictions if you would like to make the study available to participants in all courses. DurationThe amount of time, in minutes, that each study session will take. If you are setting up a 2-part study, then this setting applies to the first part of the study. This should be consistent with the duration specified on your approved participant pool request. Timeslot Usage LimitDepending on how your system is configured, you may see an item that specifies the maximum number of experimental session hours available to this study. This value is set by the administrator, and only the administrator can adjust it. To determine the current session usage for a study, go to the Add A Timeslot page for the study, and the usage will be listed there. Preparation Enter any advanced preparation a participant must do here (e.g. “do not eat 2 hours before session”). If there are no preparation requirements, leave this field as-is.Sign-Up Password (this feature might be disabled on your system)If you would like to have a special sign-up password for this study, enter it here. This is a password just for this study. Participants must know the password to sign up. This is often used in cases where the researcher wants to personally select participants, so the researcher only provides the password to the desired participants.
If you do not need a sign-up password, leave this field blank.Is this a web-based study? (this feature might be disabled on your system)If this is a web-based (online) study, choose the type of online study it is. If you have set up the study on another website, you should note the study is administered outside the system. If you want to set up an online survey study to be administered by the system, select the appropriate option.Should survey participants be identified only by a random, unique ID code?This only applies to web-based studies administered by the system, and only if participant anonymous ID codes are not already turned on system-wide. If set to Yes, participants are only identified by a unique system-assigned ID code, to protect their privacy. Participants are also notified of this when they start the survey. Once enabled, this setting cannot be changed after participants have taken the survey, as a matter of privacy protection.Study URLThe URL (web address, usually starting with http://) for your study. This is only required for web-based studies administered outside the system.
If you are setting up a web-based study outside the system, and would like the system to pass a unique identifier in the URL so you may easily identify participants, add the text %SURVEY_CODE% in the URL where you would like the identifier to be placed. This is discussed in further detail in the Web-Based (Online) Studies section of this documentation.Credits/PayEnter the number of credits and/or compensation for the study. This should be consistent with the credit specified on your approved participant pool request. Credit is calculated based on the time it takes to participate in your study (1 hour = 1 credit), and credit is rounded to the 1/2 hour (a 20 minute study = 1/2 (.5) hour credit).
For online surveys, 150-300 questions is worth 1 hour credit (under 150 = 1/2 credit).
A value of 0 is acceptable, and may be desired in cases where the study is part of a set of studies, where only the final study is credit-earning. Please see the Studies for Pay section of this documentation for more information on how to fill out this field in the case of for-pay studies. If the study has a credit value, you may specify a fractional credit value up to one decimal point of accuracy (e.g. 0.5, 1.5, etc.). If you are setting up a 2-part study, this is the value for the first part of the study.
After a study has sign-ups, you may not change the credit value of the study. However, the administrator can change the credit value, in certain situations.Is this a 2-part study?Select Yes or No if this is a 2-part study. You can only decide this when creating a study (not when editing it), and this setting may not be changed after the study is created. See “Two-Part Studies” for more information.Credits/Pay, Part 2Enter the number of credits or compensation for part 2 of the study, if this is a two-part study (the value is ignored otherwise). A value of 0 is acceptable, and may be desired in cases where the study is part of a set of studies, where only the final study is credit-earning. Please see the Studies for Pay section of this documentation for more information on how to fill out this field in the case of for-pay studies. If the study has a credit value, you may specify a fractional credit value up to one decimal point of accuracy (e.g. 0.5, 1.5, etc.).Part 2 DurationThe amount of time, in minutes, that part 2 of the study will take.Part 2 Scheduling RangeSpecify the number of days (as a range) after part 1 is scheduled, that part 2 should be scheduled. This setting only applies to two-part studies. The range may be the same value (e.g. “between 7 and 7 days”) if desired, but must be a whole number. See “Two-Part Studies” for more information.Part 2 Scheduling LeniencyIn some cases, you may want to ensure that the participant schedules the second part of the study to take place at exactly the same time (on a different date) as the first part. If so, choose Yes for this option. If there is some flexibility so they can sign up for any time within the Part 2 Scheduling range, choose No for this option.Researcher(s)Select the researcher for this study. Most likely, this is you, and your name will automatically be selected. If you are a researcher, then you may not change who the researcher is (the P.I. for the study, as well as the administrator, can change the researcher). Depending on how your system is configured, you may be able to specify multiple researchers for a study. If you specify multiple researchers, each researcher has full control over the study. The pull-down box lists only users who are researchers.Principal Investigator (this feature might be disabled on your system)Select the Principal Investigator for this study. The person you select will have full access to the study. If you see this option, then you must select a P.I.
The pull-down box lists only users who are principal investigators.IRB Approval CodeEnter the IRB approval code here. This field is displayed to the administrator to help them keep track of studies. This field may be required depending on how your system is configured.IRB Approval Expiration DateThe date when IRB approval expires. This field may not appear if your system is not configured for it. If it does appear, you must provide a valid expiration date. The system will prevent you from adding new timeslots to take place after this date, and your study will become inactive (not visible to participants) after this date. You may not make a study active if the IRB approval has expired. Only the administrator can change the IRB approval expiration date, once it has been entered. You may specify a date up to 5 years in the future.Visible to Participants?Select Yes if this study should show up on the list of studies which participants may sign up for. Ensure you have received the necessary approvals to run the study before choosing Yes. A study must be Visible and Active to show up on the list of studies which participants may sign up for.
If you select No, the study will not be visible to participants.
Some systems are configured such that only the administrator can make a study visible to participants. If that is the case, you should contact the administrator when you are ready to make the study visible to participants – and a handy form is provided on the page to do so. As a researcher, you can always make a visible study invisible to participants, but you may need the administrator to make it visible again, if so desired. In addition, if you change key items about the study, specifically the name or descriptions, the study will automatically be made invisible to participants, until the administrator re-approves it (if the system is configured this way). The reason for this is that many IRBs approve very specific language for study names and descriptions, so the administrator needs to ensure the study is in proper compliance.Active Study?Select Yes if this study is in progress. You must select Yes and the study must be Visible if you want the study to show up to participants so they can sign up for it.
If a study is Not Visible but is Active, then it does not show up (to participants) on the listing of studies, but it is accessible through other links if the participant has participated in it before and they are viewing their participation history. It will also show up on the study information page (for an individual study) when it is listed as a pre-requisite or disqualifier for a study.
The reason to select No is if the study is being kept for historical purposes, but should not show up to participants on the list of studies they may sign up for. Often, this is done so the system can enforce pre-requisites, where the inactive study is a pre-requisite for an active study.Should the Researcher receive an email notification when a participant signs up or cancels?If set to Yes, the researcher for this study will receive an email notification whenever a participant signs up, or cancels their sign-up, for this study. The email notification will be sent to an email address based on the information the researcher has provided. See the Email Address Options section of this documentation for more information on how the email address is determined.
If set to Yes, researchers will also receive a notification if the system is in lenient pre-requisite enforcement mode and a participant cancels a study that was a pre-requisite for the current study. Read the section on Pre-Requisites in this table for more information about this situation.
Emails are sent to all researchers specified for the study, unless a specific researcher is assigned to the timeslot that the email notification is being sent about. See Timeslots Linked to Specific Researchers for more information.Researchers at Timeslot-LevelIf set to Yes, it will be possible (but not required) to assign a specific researcher (from the list of researchers for the study) to a timeslot. If set to No, then it is assumed that all researchers (assigned to the study) are responsible for all timeslots. See Timeslots Linked to a Specific Researcher for more information. This option only appears if the system is configured to allow multiple researchers per study.Automatic Credit GrantingIf set to Yes, timeslots that are more than a specified number of hours old and still in the Awaiting Action state will be changed to a credit grant. The check for timeslots in this situation is made only once per day. If an automatic credit grant is done, you may still change it later if necessary.Can a participant sign up for this study more than once? (this feature might be disabled on your system)If you would like to allow participant to sign up (and receive credit) for your study more than once (at different times), choose Yes. Otherwise, choose No.
If No is chosen, participants may only sign up for the study more than once if they previously failed to show up for the study.Private CommentsThis is an optional area where you may enter any comments or notes about the study, which are only visible to the researchers for this study.Participant Sign-Up Deadline (this feature might be disabled on your system)Enter the deadline before the study is to occur that the participant may sign up, in whole hours.Participant Cancellation Deadline (this feature might be disabled on your system)Enter the deadline before the study is to occur that the participant may cancel their existing sign up, in whole hours. Generally the cancellation deadline should be shorter than the sign-up deadline, so participants can easily cancel an accidental sign-up.
Once you have filled out the appropriate information, save it and the system will be updated immediately with the information. Your next step is likely to add timeslots (sessions). See the Working with Timeslots section of this documentation for more information.
If you need to update this study, see the following Updating a Study section of this documentation. If you would like to add participation restrictions based on pretest responses, you can do so when you update the study (see Pretest Participation Restrictions).
Updating a Study
You may update any of your studies at any time. To do so, choose My Studies from the top toolbar, and you will see a list of your studies. Click on the desired study, and choose the Change Study Information link. You will see a form similar to the one you used to add the study, but a few options may no longer be changeable depending on the status of the study (e.g., if participants have already signed up for it). The fields shown are all the same as when you added the study. See the Adding a Study section of this documentation for an explanation of those fields.
The changes you make will be will be take effect immediately after they are saved. If administrator approval is required before a study is made visible to participants, and the name, description, or eligibility requirements of the study are changed, then the study will require re-approval by the administrator before it is again visible to participants. The reason is that many IRBs are quite strict about a study’s wording, so the administrator must look over any changes.
If you need to change the credit value for a study, and there is no option to do so, this means the study already has at least one participant signed up for it. You cannot change the credit value when a study is in this situation because there is no easy way to handle past credits for the same study (e.g. should old credit grants for the same study be adjusted to reflect the new credit value, or kept the same?). If the study is nearing the end of its run, and variable credit granting is enabled, then the easiest solution is to grant the new credit value to participants who sign up in the future. If you prefer that the credit value is changed for the entire study, contact the administrator, who can make the change for you under certain conditions.
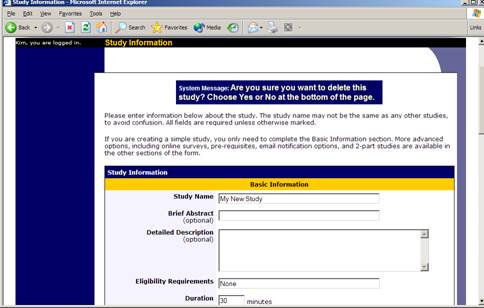
Deleting a Study
You may delete a study only if participants have not signed up for it. If you need to delete a study which already has sign-ups, you should make it Inactive instead, if you do not want it to be visible to participants. You may not delete a study with has sign-ups, so the option will not be presented. If you want to delete a study that has sign-ups, please contact the administrator. The administrator can delete a study with sign-ups, but only if the sign-ups are all without credit values (this usually occurs when study participation history from a previous semester was retained).
Figure 5- Deleting a Study
To delete a study, choose My Studies from top toolbar, click on the desired study, then choose the Delete Study option. You will see a confirmation page. Choose Yes (at the bottom of the page) to delete the study. Once a study is deleted, it cannot be restored, so use this feature very carefully. If you delete an online survey study, the survey will also be deleted.
Pretest Participation Restrictions
The system contains an online pretest that participants may complete. You may place participation restrictions on your study based on pretest responses. Participants are unaware that such restrictions are placed on the study. These restrictions are never listed to them. If they do not qualify to participate in a study because they do not meet the pretest participation restrictions, then the study will simply not be listed to them. This is important to note – participants never know why a study was or was not listed to them, because they are unaware of the pretest restrictions.
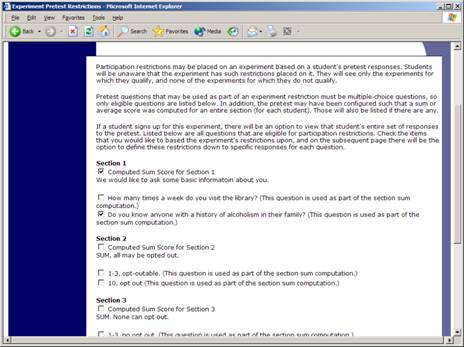
You may restrict a study on any question or questions on the pretest that allowed for a multiple-choice answer where only one choice could be selected. You may also restrict a study based on a computed section sum or average score for a participant, if the pretest was set up in such a manner. You may restrict to one choice or many choices for any question. If you restrict on multiple questions, it is the same as a logical “AND.” For example, if you setup the pretest restrictions so that participants must have answered “Yes” to a “Do you wear glasses?” question and “Blue” or “Grey” to “What color are your eyes?”, then they must meet both requirements to participate. In other words, only participants who wear glasses and have either blue or grey eyes are eligible. There is no support for a logical “OR” restriction across multiple questions.
Figure 6 – Pretest Restrictions Question Selection
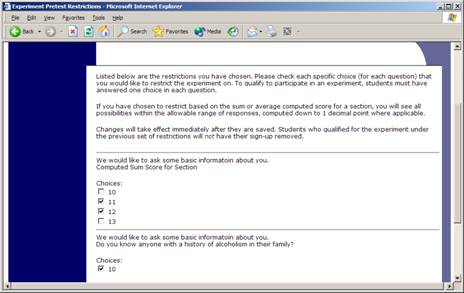
To set participation restrictions, view (do not choose edit) your study and choose View/Modify Restrictions. You will see a list of eligible questions which you may use for your restrictions. If the study already has some restrictions, those will be checked, and you will see how many participants currently meet the restrictions. Choose the questions you would like to restrict upon (and keep the existing checked restrictions checked, unless you want to remove that restriction), and click on the Set Restrictions button. On the subsequent page, you can select each value that is acceptable for each question you have chosen. Once you have selected all the acceptable values, save your changes and they will take effect immediately. It is important to note that if you change the restrictions, it will not remove the study sign-ups for participants who qualified under the previous set of restrictions. For this reason, you should probably decide on your restrictions before making the study available to participants.
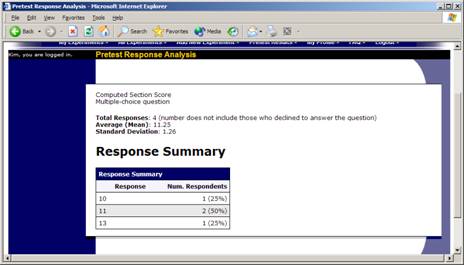
If you have restriction requirements where you would like to restrict participation to a percentage of the population (for instance, the responses that were chosen by the top 25% of people) but you are not sure which responses meet this requirement, you can use the pretest response analysis feature to determine the valid responses. See Pretest Response Analysis for more information. You may also use Analyzing Pretest Responses to get an idea of how many participants are potential candidates for participation in your study, based on a specified set of restrictions.
Figure 7 – Pretest Response Restrictions
Viewing Your Studies
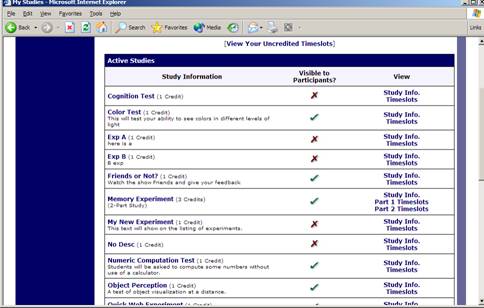
To view your studies (and not the studies of others), choose the My Studies option on the top toolbar. The system will list all your studies in alphabetical order by study name, grouped by studies that are active, then inactive studies.
Figure 8 – Your Studies
Viewing Other Studies
To view all studies that are visible to participants, choose the All Studies option from top toolbar.
You will see a list first of all Active studies. These studies will show up to participants on the list of available studies. The next group of studies (if there are any) are Inactive studies. These will not show up on the list of available studies (to participants), but participants can access information about these individual studies on links from the page with their progress (if they participated in the study) or if another study has the Inactive study listed as a pre-requisite or disqualifier.
Online Survey Studies
Introduction
The system allows you to set up an online survey as a study. Participants who sign up for the study will be asked to immediately complete the survey. Upon their completion of the survey, they will be granted credit automatically by the system. You may then analyze their survey responses on an individual basis, or download the raw data across all participants who completed the survey, for further analysis. There is a slight chance that you may notice a discrepancy in the number of responses when analyzing a single question compared to downloading the entire set of responses. This can occur if a participant is currently taking the survey, but has not completed it. Their data is included in the single-question analysis (when available) but not in the full download of responses, as there is not a full set of data for an in-progress participant. The system will prevent participants from completing the survey more than once, so there is no risk of duplicate entries for the same participant.
To comply with regulations, the participant is given the opportunity to withdraw from the survey at any time. If they withdraw, they are taken to a form where they can submit any comments (this is optional), and their withdrawal is then noted and all their responses are deleted. The researcher receives an email when this occurs, with some other information, including how much time was spent on the survey, and how many questions were answered.
There is a space to provide closing text, which is displayed to participants after they successfully complete the survey (after they have saved all their responses and cannot go back). This is an ideal place to include any relevant debriefing information.
The online survey feature is rather complex due to the many features it contains. In addition, your ability to modify the survey after participants have started to take the survey is quite limited. Because of this, you should plan out your survey well in advance, to make sure it is finalized before you make it available to participants. You may find it helpful to plan out the survey on paper before entering it into the system.
A survey may have an unlimited number of sections and an unlimited number of questions per section, though we recommend limiting surveys to fewer than 300 total questions and 15 sections, to reduce participant fatigue. This can be an issue since they must complete the survey all at once. The system will make participants aware that their session may time out because of inactivity.
Questions may be free-entry (requiring the participant to type in an answer) or multiple-choice (pick only one or pick many from the list of choices). You may also specify that the system computes a participant’s results for an entire section, as either a section sum or average score. This computed sum or average can be computed only for numeric, multiple-choice (pick only one) questions in the section. Such a computation is often useful when a participant’s aggregate score is more important, such as with a depression battery.
For free-entry questions where the participant is asked to enter a free-form text answer, the size of the entry field (as it is displayed) cannot be changed or be resized. Participants may enter up to 255 characters in their response.
Sections can be displayed in a specified order or random order, or a combination of both. Every section may contain introductory text introducing the section, and the survey itself may also have introductory text introducing the survey, as well as closing text that is displayed upon completion of the survey. You may specify that questions within a section are displayed in random order. If you do not specify random ordering for questions in a section, then the questions will be displayed (in each section) in the order they were entered and cannot be reordered later. Multiple choice questions can have their choices displayed in the entered order or random order (this is specified on a per-question basis). Multiple choice question choices can be displayed horizontally (across the page) or vertically (down the page).
If you would like to see how the survey will be displayed to participants, that can easily be done while setting up the survey. Next to each section (in the section list), there will be a Preview Section link so you can view the section exactly as participants would see it. You may also view how the introductory and closing text will appear to participants, by choosing the Preview Introductory Text and Preview Closing Text options which appear on the Section List page.
Note that there is a bug in Internet Explorer 5 on the Macintosh platform that may result in a slow response for participants when they are using this specific web browser/computer combination and completing survey sections with many choices. Microsoft has discontinued support for Internet Explorer on the Mac, so this bug will not be fixed. The other browsers on the Mac (Firefox, Safari, and Mozilla) all work perfectly, and there are no problems for Windows users with any browser. The specific problem occurs only rarely, but can occur.
It is important to realize that while the survey feature is quite extensive, there are some things it does not support. It cannot do timings, where the speed of a participant’s response (typically in milliseconds) to each question needs to be measured. It also cannot do branching, where the questions being presented to a participant will vary depending on their answer to other questions within that same survey (an example being asking a question like “Do you smoke cigarettes?” and then presenting a different set of questions based on their response). The reason advanced features like this are not currently supported is that our software is primarily a subject pool software product that happens to have an online survey feature within it. We asked our customers which features in an online survey were most important to them, and those features exist within the product. There are numerous products on the market specifically for doing online surveys (with no subject pool component), which contain more advanced features, and can easily be linked to from the Sona Systems product by setting up your study as an online study conducted outside the system.
Creating or Modifying a Survey
To create a survey, first create an online survey study. After you create it, you will see the option Update Online Survey when you view the study information. You may not create 2-part online survey studies.
It is important to note that while you are making changes to a survey, it is deactivated so participants may not participate in it. When you go to the Finalize Survey option after you have finished your work with the survey, you will have the opportunity to save your changes and make the survey active (available to participants) or inactive (not available to participants). You should always choose the Finalize Survey option when you finish your work with the survey, as the system saves all changes and computes some other important data when you finalize the survey. If you would like to continue work on the survey at a later date, you should still choose the Finalize Survey option to ensure your changes during the current session are properly saved (most likely you would choose to keep the survey inactive in this situation).
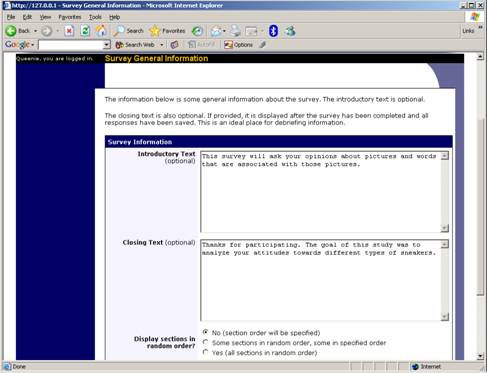
General Survey Information
Choose the General Information option (if creating a new survey, this is the first page you will see) to provide some basic information about the survey. The fields are explained on the following page.
| Field | Description |
| Introductory Text | Optional. Provide a set of text that will be displayed when a participant starts the survey. You may include HTML in this area, but please be sure you know what you are doing. If you would like to add a carriage-return (paragraph break), simply type in “<p>” (without the quotes). When you first create the online survey, the system will automatically insert some basic default text in this area. You may change any of this text as much as you like – the system will not change it back later as long as you save your changes. This is a good place to place any text for informed consent purposes. To see how the text is displayed to participants, saving it, then go to Preview Introductory Text, which is available from the Section List page. |
| Closing Text | Optional. Provide a set of text that will be displayed after a participant completes the survey and saves their responses. This is an ideal place for debriefing information. You may include HTML in this area, but please be sure you know what you are doing. If you would like to add a carriage-return (paragraph break), simply type in “<p>” (without the quotes).You may view how this text is displayed to participants by saving it, then going to Preview Closing Text, which is available from the Section List page. |
| Display sections in random order? | You may specify a specific ordering for sections, have the system randomize the order for all sections, or specify the order of some sections, and a random order for other sections. If you specify the order for only some sections, you may also specify, for each section, whether it should be displayed before or after the random-ordered sections. The system does not keep track of the random ordering for sections for each participant. |
| Participant response review/change | If set to Yes, then participants may review and change their responses just before they complete the online survey. They are given this opportunity after they complete the last section of the survey, and before any closing text is shown. At this point, they can see all their responses and go back to any section to change their responses. Once they complete the online survey, they cannot go back at a later date and change any responses. It may be useful to set this option to No (so that they are not allowed to review or change their responses at the end of the survey) if sections are somehow dependent on one another, such that allowing participants to change their responses may affect the integrity of data collection. |
This is a good place to place any text for informed consent purposes. To see how the text is displayed to participants, saving it, then go to Preview Introductory Text, which is available from the Section List page. Closing Text Optional. Provide a set of text that will be displayed after a participant completes the survey and saves their responses. This is an ideal place for debriefing information. You may include HTML in this area, but please be sure you know what you are doing. If you would like to add a carriage-return (paragraph break), simply type in “<p>” (without the quotes).
You may view how this text is displayed to participants by saving it, then going to Preview Closing Text, which is available from the Section List page.Display sections in random order?You may specify a specific ordering for sections, have the system randomize the order for all sections, or specify the order of some sections, and a random order for other sections. If you specify the order for only some sections, you may also specify, for each section, whether it should be displayed before or after the random-ordered sections. The system does not keep track of the random ordering for sections for each participant.Participant response review/change If set to Yes, then participants may review and change their responses just before they complete the online survey. They are given this opportunity after they complete the last section of the survey, and before any closing text is shown. At this point, they can see all their responses and go back to any section to change their responses. Once they complete the online survey, they cannot go back at a later date and change any responses.
It may be useful to set this option to No (so that they are not allowed to review or change their responses at the end of the survey) if sections are somehow dependent on one another, such that allowing participants to change their responses may affect the integrity of data collection.
Figure 9 – Survey General Information
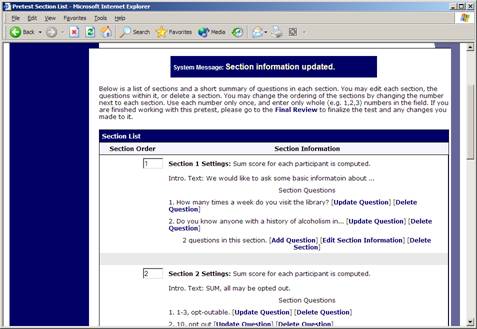
Section List
Depending on if you are adding or editing the survey, you will be taken to the list of sections for the survey. Here, you can change the order that sections will be displayed (if you have not enabled full random section ordering for the survey), and see a quick review of each question. From here, you can add a question to any section and edit any question. To add a new section, choose the Add a New Section choice at the bottom of the page.
Figure 10 – Survey Section List
If you have enabled partial random section ordering, then you can specify a section order for the sections you would like to be displayed in a specified order. For the sections you would like to be displayed in random order, leave the Section Order area blank. When you specify a section order, use each number once, and use the numbers 1-98 if you would like the section to be displayed before the random-ordered sections, and the numbers 100-199 if you would like the section to be displayed after the random-ordered sections. Be sure to use each number only once, or leave the number blank to make the section part of the random-ordered section. Sometimes it is useful to use partial random ordering if you want to ask basic (e.g. demographic) information in the first few sections, while asking more analytical questions in the random-ordered sections. Likewise, you may want to ask about the previous random-ordered sections after they are completed. There is also a Preview Section option so you may preview the survey as participants will see it.
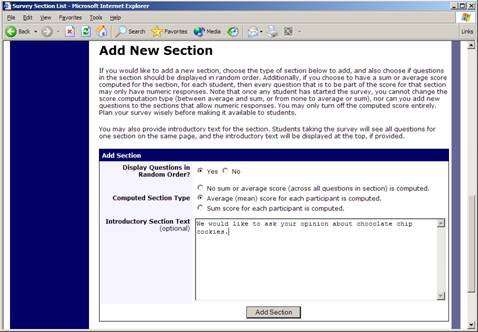
Adding or Modifying a Section
To add a new section, choose the Add New Section link at the bottom of the Section List page. To modify an existing section, choose the Edit Section Information link next to the section you would like to modify, from the Section List page.
Figure 11 – Add New Section
There are three pieces of information you may provide for each section. First, you may specify some introductory text to be displayed at the beginning of the section. When participants take the survey, they view one section at a time, and all the questions in that section. The introductory text may be helpful in explaining the purpose or topic of the questions in that section. You may include HTML in this area, but please be sure you know what you are doing. If you would like to add a paragraph break, simply type in “<p>” (without the quotes).
You may choose to have questions for that section displayed in random order or the order in which they are entered. You may also choose to have the system compute a section sum or average (for each participant) for the section. These scores are computed only for all the multiple-choice, numeric questions in a section. Think carefully about setting this value, because you are very limited in your ability to change it after participants have started to take the survey. Computed section sums or means are often useful when all the questions are using a one-dimensional scale (1-5, for example), but a participant’s average score response to the section is more useful to account for their outlier responses.
Depending on if you are editing or adding a section, after you save your changes, you will be taken to a page to add a new question or to the section list.
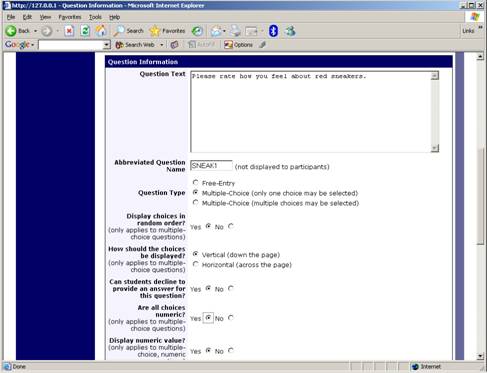
Adding or Editing a Question
To add or edit a question, choose the appropriate choice from the Section List, next to the desired section. Depending on the state of the section and if participants have taken the pretest, some options will be automatically set for you, and not changeable. All fields must be filled out.
| Field | Description |
| Question Text | The text for the question. This will be displayed above each question’s choices. |
| Abbreviated Question Name | An 8-character label for the question. This is not displayed to participants and is used to name the columns that appear when the data is exported in CSV format. Many statistical analysis programs limit column names to 8 characters. |
| Question Type | Multiple Choice (select one), Multiple Choice (select many) or Free Entry. If the section has a computed average or sum, you may be limited in your ability to add Multiple Choice (select one) non-numeric questions. Free entry choices may contain a response up to 255 characters in length. |
| Display choices in random order? | If set to Yes, the choices for this question will be displayed to participants in a random order. This option only applies to multiple choice questions. |
| How should the choices be displayed? | Horizontal (across the page) or vertical (down the page) are the two options for how the choices for a multiple-choice question should be displayed. Often, the horizontal display is effective when the question involves a scale. This setting applies only to multiple-choice questions. |
| Can participants decline to provide an answer for this question? | If set to Yes, participants will see a choice below this question that allows them to opt out of answering the question. If set to No, they must answer the question. |
| Are all choices numeric? | If set to Yes, all choices must be numeric whole numbers (e.g. 1, -2, 10). This only applies to multiple choice questions. Sections with a computed section sum or average may not contain non-numeric multiple choice (select many) questions. |
| Display numeric value? | If set to No, the numeric value of the choice is not displayed to participants. This only applies to numeric, multiple-choice questions where each choice includes both a numeric value and associated text (e.g. “5 Strongly Agree”). This is useful when running a reverse scale. |
If the question is a multiple choice question, you must also fill out the choices section that is part of the same form. A minimum of 1 choice must be provided for the question. Each choice may be up to 255 characters in length. If the question is specified as numeric, you must provide a numeric choice, and that choice must be a whole number (e.g. -5, 1, 0, 349). If you would like to provide descriptive text to accompany each numeric choice, you may do so by typing in the numeric choice and leaving one blank space after it, then typing in the descriptive text, for example: “5 Strongly Agree”. If you provide descriptive text along with the numeric choice, then you have the option of hiding the numeric value from participants, by choosing No to the “Display numeric value?” setting for the question. This is useful in reverse scale or other situations where the numeric value for the choices is not important.
Figure 12 – Edit a Question
Copying a Section
You may copy a section from any of your own surveys into your current survey, by choosing Copy From an Existing Section in the Section List. You will see a list of eligible surveys to copy from. After you choose which survey to copy from, you will see a list of eligible sections in that survey. Once you choose a section, it will be copied into the survey you are currently editing.
Copying a Question
For multiple-choice questions, it is often the case that many questions have the same measures (choices). To make entry of similar questions easier, you may use the Copy Question feature to copy a question. Such an option is only available for multiple choice questions. To copy a question, select the question you would like to copy (when viewing the Section List). You will then be taken to a list of sections where you may copy this question to. After you decide which section you would like to copy the question to, you will be taken to a page where you can make any final changes to the copy of the question before saving it.
Saving Your Changes
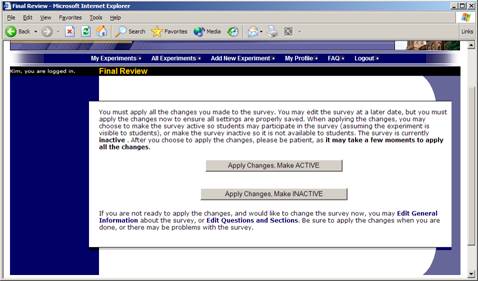
When you are done, you should go to the Final Review and save your changes. Even if you plan to do further editing of the survey later, it is imperative that you go to the Final Review step, as the system needs to save certain special changes and make some computations. If you do not want participants to participate in the survey yet, you can save changes but keep the survey inactive. When you save your changes, it may take a moment to save all the changes, as the system is performing a number of computations on the survey. Please be patient, as these computations are done to make performance for participants as fast as possible.
Figure 13 – Saving Survey Changes
Deleting a Participant’s Survey Responses
On rare occasions, you may want to delete a participant’s survey responses, usually so they can participate again. To do this, cancel their sign-up and that will delete their responses. Note this will also remove any credit they earned. See Manual Cancellation for more information.
Analyzing Survey Responses
You may analyze a specific survey question on-screen by selecting the survey and choosing the Analyze Survey Responses option. From there, you may choose a specific question and view or download the response data.
More likely, you will want to analyze the survey data across all questions. In this case, you should choose the Download Survey Responses option.
To successfully analyze the data, you will need to download 2 sets of data. The first is the question key, which lists a unique numeric identifier for each question, along with the question text and abbreviated question name. It also includes the section number each question was in. Note the section number listed is merely a unique identifier for each section, and has no correspondence to the order in which sections were presented. Also note that section mean and sum values, if calculated, will normally be at the end of the list of questions. This data is in CSV format, and is available by clicking the Download Question Key link on the Download Survey Responses Page.
After you download the question key, you can download the survey data. The system may require that you download the data in sections if there is too much data to be downloaded as one file. The data is in CSV format, and is presented as one row per respondent, with each of their responses in a different column. The first row includes the column headings, and the column heading maps to the abbreviated question name. Since there is no facility to specify an abbreviated question name for the computed section sum/mean item, the system automatically assigns a unique name to those columns. The naming convention is SECXXX where XXX maps to the system’s internal section identifier for that section (section_id). You can use the question key to determine which question maps to which column. The data also includes the exact time the participant started and completed the online survey. You can use any common data analysis tool to compute how long it took the participant to complete the survey, using this information.
There will be an option to include only the numeric response portion of questions that are multiple-choice with numeric choices. This is useful if the numeric question was set up with associated text, but that text should not be included in the analysis file. One example would be where the response selected was “5 Strongly Agree” but only the number 5 (and not the associated text) is useful during analysis.
If your survey has a lot of questions (and thus columns in the output file), you may have trouble loading the data with some spreadsheet programs, which are not equipped to handle such large datasets. Your best option is to use a full-feature statistical analysis packages, like SPSS or SAS. Those packages handle large CSV-format data imports with ease.
Frequently Asked Questions About Online Surveys
Is there a way to post images, graphics, or videos in the online survey, so that participants can respond to questions referring to the image or video?
Yes. You will need to have the ability to post images on a webserver (most likely at your school), and know a small bit of HTML. Let’s say your graphic is located at http://www.yourschool.edu/myimage.gif
Put this in the place where you want the image to be displayed:
<IMG SRC=”http://www.yourschool.edu/myimage.gif”>
If you’re unfamiliar with HTML, you may want to ask someone for assistance with this.
You can use a similar process to post videos, but you’ll need to ask the person in charge of the webserver where you post the video for the best method (in HTML) for linking to it, as there are a few different methods to link to videos.
Can certain sections be presented for a specified period of time (e.g. 60 seconds)?
No. There are technical limitations and inaccuracies in how web browsers interact with web servers, as well as internet latency that make this difficult to do, so this feature is not offered.
Can I make an online study a two-part study?
Online studies cannot be two-part studies, and the reason has to do with what two-part studies are intended for. They are used when the researcher needs to ensure a fairly strict separation between each part. For example, in memory research, a researcher may want to set up the two parts exactly 14 days apart.
The issue with online studies is they are not scheduled to take place at a certain date and time, so it’s impossible to enforce any kind of strict separation between each part.
If the goal is just a follow-up to a study, then the time separation probably isn’t very important. In that case, the solution is to set up two studies, and set the first study as a pre-requisite for participation in the second study.
Is there a method to upload a survey into the system (from Word, Excel, etc.)?
For online survey studies, there is no method to upload the data. We considered this, but there is no standard format for survey data representation, so there was no format we could really support for uploading.
In the online survey feature, there is the ability to copy questions and sections, which may make it easier to set up the survey if many questions have the same sets of choices, for example.
When a participant is taking an online survey study, when is their sign-up actually recorded in the system?
The system will not record completion (or even sign-up) of an online survey study until it is actually completed (or if the participant chooses to withdraw early). Participants are shown the closing text as the last step of the online survey study — just after their responses have been saved and their sign-up is recorded.
When they start the online survey study, it is noted to participants that their responses are not fully saved until they complete the study, and only then will they be properly recorded. This is for both technical and IRB reasons.
Working with Timeslots (Sessions)
Timeslots (also referred to as Sessions) are the available times when a participant may participate in the study. If you are setting up timeslots for a web-based study, please read the section in this documentation on Web-Based (Online) Studies for some special information.
Timeslots allow you to specify a date, time, location, maximum number of participants, and researcher for a session.
Timeslot Usage Restrictions
If enabled on your system, you may find there is a limit to the amount of time available for scheduling timeslots. This usage is computed by adding up all the past timeslots where credit was granted, and then adding all timeslots in the future, regardless of credit status. You may find that the usage goes down over time, as time progresses and timeslots that were in the future had no participants signing up for them. The usage and limit is listed whenever you add a timeslot, if usage restrictions apply. It may also be listed when you view your profile, depending on how your system is configured.
Timeslots Linked to Specific Researchers
If your system is configured to allow multiple researchers per study, you will also have an option to link timeslots to a specific researcher. This is done primarily for organization purposes, and has no effect on who can view and modify the study, or any timeslots for that study.
This feature is useful when there are a number of researchers running a study, and researchers are responsible for running specific timeslots. If a timeslot has a specific researchers linked to it, then only that researcher will be listed as the contact point when a participant receives any emails related to their participation in that timeslot. Finally, only the researcher connected to that timeslot receives related notification emails, such as participant cancellation notification, and reminder emails (assuming such emails are enabled).
It is also possible to have some timeslots where a specific researcher is linked to it, and others where all researchers (who are assigned to the study) are responsible for the timeslot. If a researcher is removed from a study, then any timeslots that were linked to them for that study will be changed so all researchers (for the study) are now responsible for those timeslots.
To use this feature, the system must be configured to allow multiple researchers per study. Then, the study itself must be configured to allow researchers to be linked to specific timeslots. Finally, the study must have more than one researcher connected to it.
Creating Timeslots
To add a timeslot for a study, you must first choose the study that you would like to add a timeslot for. To view your studies, choose the My Studies option on the top toolbar. Click on the desired study, and choose the Timeslots choice.
You will see a list of any existing timeslots, and the Add A Timeslot option at the bottom of the page. Click on Add A Timeslot.
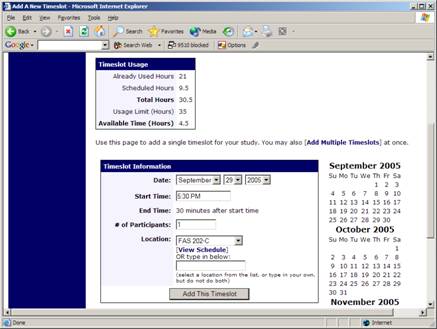
Figure 14 – Adding a Timeslot
The following table lists the information you may enter about a timeslot, along with an explanation. All fields are required.
| Field | Explanation |
| Date | The date for the timeslot. |
| Start Time | The time for the timeslot. A sample time will be provided. If you want to change the time, please use the same format as the time you see presented. Note in particular how “a.m.” and “p.m.” are handled (if such a format is enabled on your system). |
| End Time | The time when the timeslot will end. This is computed automatically based on the duration you entered when you set up the study. |
| # of Participants | The number of participants for this timeslot. This limit is not visible to participants. They will only see whether the timeslot is full or not. |
| Location | The physical location where the study will take place, for this timeslot. It will be automatically filled with the location of the previous timeslot, when available, to ease in data entry. |
Depending on how your system is configured, you may see a list of pre-configured locations. You may choose any of those locations and click on View Schedule to see the schedule for a location. The system will automatically prevent you from adding a timeslot using a location that is already in use at the time you try to schedule the timeslot. If you do not see the location in the list that you plan to use, you can simply type in the location in the text field below it.
The location field does not apply for web-based studies.ResearcherThe researcher assigned to this specific timeslot. The list will contain a list of all researchers for the study. Choose ALL if all researchers (for the study) should be assigned to this timeslot. See Timeslots Linked to Specific Researchers for more information.
To ease data entry, the system will automatically fill in the date, time, and location based on the ending time of the last timeslot for this study. If applicable, your current timeslot usage will be listed, and you will be prevented from adding a timeslot that would exceed your timeslot usage time limit. A convenient calendar is provided next to the form, and you can click on any date and that date will be transferred to the form.
If you add a timeslot such that there is another timeslot (for any study) that occurs in the same time, at the same location, you will receive a warning (but the addition will be allowed). If you add a timeslot that will take place outside of normal hours (for example, at 1:00am), they system will provide a warning but it will allow it to be scheduled.
If you are running a web-based (online study), you should create a single timeslot with the participation deadline equal to the last day you would like to run the study. For number of participants, specify the maximum number of participants who may participate.
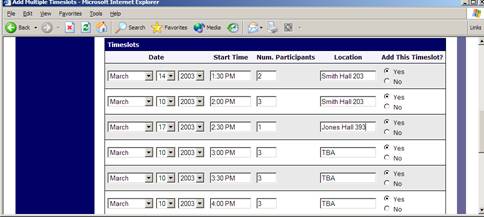
Creating Multiple Timeslots
If you would like to add multiple timeslots at once, choose the Add Multiple Timeslots link. You may choose to add a specified number of timeslots, or copy the timeslots from another week to a specified week. If you choose to copy, the system will copy the time, location, and number of participants for the specified week to the desired week, for each day of that week (starting with Monday).
If you choose to create a specified number of timeslots, you can choose the number of timeslots you would like to add, the start time and date, and the amount of time between each timeslot (to allow for breaks). You also may specify that timeslots that would occur outside normal business hours be shifted to the next business day. On the subsequent page, you may change any of it to deal with special cases. Timeslots that you attempt to add, that either have errors or would result in exceeding the timeslot time usage limit, will not be added. This feature is not available for web-based (online) studies, as web-based studies rarely have more than one timeslot.
If you would do not want to add a specific timeslot that is listed, choose No in the Add This Timeslot? Column.
Figure 15 – Creating Multiple Timeslots
Modifying and Deleting Timeslots
To modify or delete a timeslot for a study, you must first choose the study that you would like to deal with. To view your studies, choose the My Studies link from the top toolbar. Choose the Timeslots option in the timeslots column for the desired study. You will see a list of all recent timeslots. Recent timeslots in the past with no participants signed up will not be displayed. To work with timeslots more than a few days old and to see all timeslots, you will see a link to view all timeslots for the study. Select the timeslot you would like to deal with, and click the Modify button.
If the timeslot has no participants signed up for it, you will see a Delete button. You may not delete a timeslot that has participants signed up for it. If you would like to delete the timeslot, click the Delete button, and you will see a confirmation page. Choose Delete again to delete the timeslot.
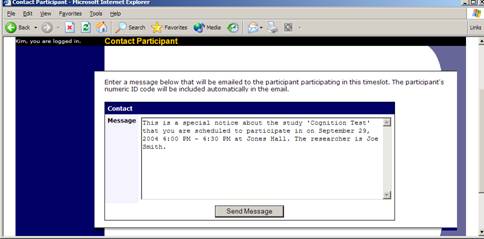
If you would like to modify the timeslot, modify the desired information and click the Update button just below the timeslot information. It should be noted that participants will not be notified (by email) of any changes you make to the timeslot, so you should contact them if information needs to be passed on to them (a link is provided on the same page to do so). If you change the date or time of the timeslot, you will be warned that this was changed in case the change was unintended. If you change the maximum number of participants to a lower number than it was before, the system will not cancel the sign-ups for any participants who are over the new limit of participants. Generally, researchers only update timeslots with sign-ups to update the location, if it was not available when the timeslot was originally created.
If the study (or researcher) is subject to timeslot time usage restrictions, the system will enforce them and prevent you from increasing the number of participants in a timeslot if that would result in exceeding the timeslot usage limit.
Deleting Multiple Timeslots
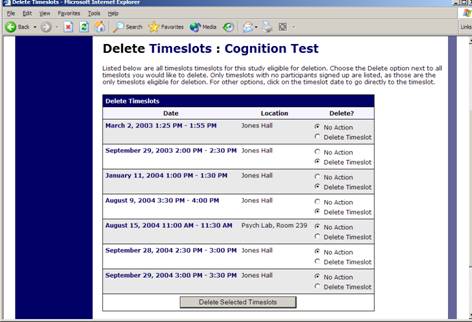
If you would like to delete multiple timeslots at once, you may do that as well. Such a feature is only available for timeslots which have no participants signed up. To do so, select the desired experiment and choose Timeslots. At the bottom of the Timeslots page, you will see a Delete Multiple Timeslots option. The option may not appear in certain cases where such an option is not available because of a lack of available timeslots to delete.
After going to that page, you will see a list of timeslots eligible for deletion. Choose the timeslots you would like to delete, and choose Delete Selected Timeslots to proceed.
Figure 16 – Delete Multiple Timeslots
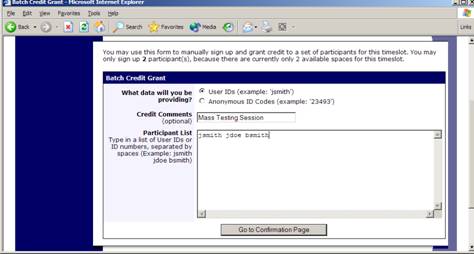
Manual Sign-Up
If enabled on your system, you may manually sign up participants for your study. There are a number of situations where this is desirable. If the participant happens to show up for a timeslot they were not signed up for, and you elect to let them participate, you can sign them up on the spot for the timeslot. The participant in many cases cannot sign up on their own in this situation, because the sign-up deadline has passed. You may also sign up a participant for a study that has already occurred, if necessary.
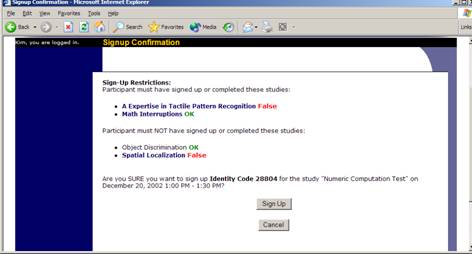
Also, a manual sign-up overrides any restrictions you have placed on the study (e.g. pre-requisites), though you will be warned if you are overriding any restrictions.
If the system is configured as such, the participant will receive an email when you sign them up for a study. In that case, you are also given the option to enter comments to be included in this email that may better explain to the participant why they were signed up. You may only sign up participants for your own study.
To sign up a participant for a timeslot, you must first find the desired study and timeslot. To view your studies, choose the My Studies option from the top toolbar. Click on Timeslots for the desired study, then select the timeslot you would like to deal with, and click the Modify button.
At the bottom of the page, you will see a Manual Sign-Up option, if it is enabled. Type in the participant’s User ID (you may have to ask them for this) and click Sign Up. If enabled, you may also choose to sign up a participant using their unique ID code. You may also have the choice to enter their last name and choose from a list of participants. In all cases, after submitting the form, you will see a confirmation page that also lists any restrictions on the study. Choose Sign Up to complete the sign-up.
Figure 17 – Manual Sign-Up Confirmation
If you are subject to timeslot time usage restrictions, the system will enforce them and prevent you signing up a participant in the timeslot if that would result in exceeding your timeslot usage limit.
If you are doing a manual sign-up for a two-part study, you must do a manual sign-up for each part separately. The system will overlook the scheduling range restrictions as well.
You cannot use the manual sign-up feature for online survey studies, because the sign-up for the study is integrated with the administration of the survey.
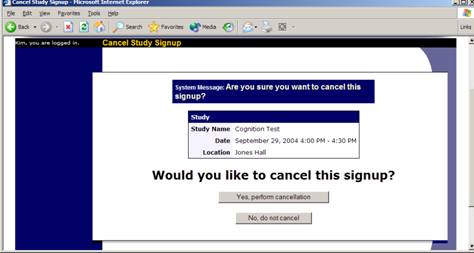
Manual Cancellation
If enabled on your system, you may have the opportunity to cancel a participant’s sign-up. You may only cancel sign-ups that are in a No Action Taken state. To cancel a sign-up, find the desired timeslot and participant, and click Cancel next to their name. The participant will be emailed an email about the cancellation, along with a confirmation code, and their sign-up will be immediately cancelled.
Figure 18 – Manual Cancellation
Viewing the Participant List
To view the list of participants who have signed up for your study, you must first select the study and timeslot you wish to see. To view your studies, choose the My Studies option from the top toolbar. Click on the Edit link in the timeslots column for the desired study then select the timeslot you would like to see, and click the Modify button.
The list of participants, along with their email addresses, will be listed. If ID codes are enabled, you will only see an ID code and no name or email address for each participant.
Figure 19 – Modifying a Timeslot / Participant List
Viewing Pretest Responses
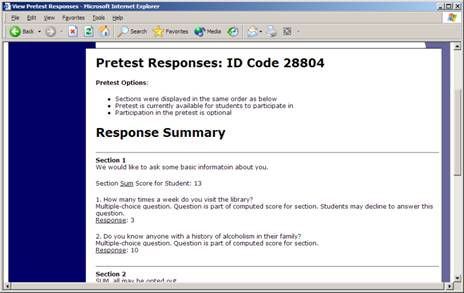
To view participant’s pretest responses, you may click the link next to each participant’s name (or ID code) when you view the information for a timeslot. If you would like to download the pretest data for all participants in your study, choose the Download Pretest Responses option after clicking on your study. That will allow you to download all the data at once, in CSV (comma-separated) format, for further analysis. If you would like to analyze responses in aggregate (across all participants in the system), see Analyzing Pretest Responses in this documentation.
Figure 20 – Viewing a Pretest
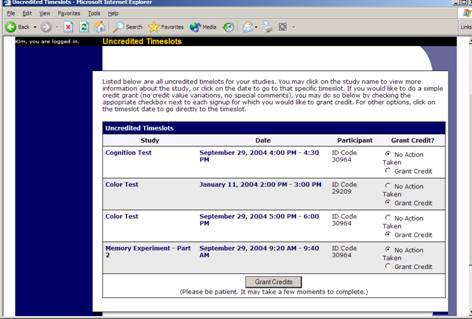
Granting or Revoking Credit
At the completion of a session, you should promptly deal with the participants, in the system, to ensure proper credit grants. The reason for the prompt handling of this situation is in the event your study is a pre-requisite for another study, and a few other situations. You do not want to hold up other studies that are waiting on your response to the study you just ran.
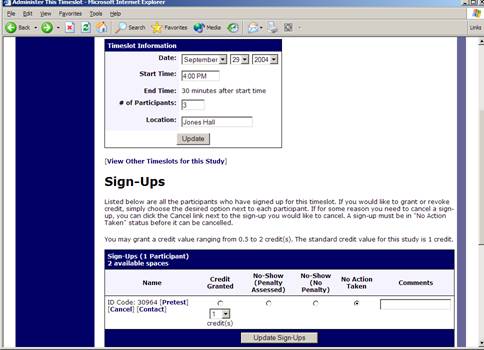
To grant or revoke credit for a timeslot, you must first find the desired study and timeslot. To view your studies, choose the My Studies option from the top toolbar. Click on the Edit link in the timeslots column for the desired study then select the timeslot you would like to see and click the Modify button.
You will see a list of participants, identified either by name or ID code. If the participant properly participated in the study, click the Credit Granted button next to their name. If the participant did not appear for the timeslot, you may choose to mark their no-show as excused or unexcused. Generally, excused no-shows are granted for extenuating circumstances, like if the participant was involved in a car accident on their way to the appointment. An unexcused no-show is generally used when the participant did not show up and had no reasonable excuse.
If desired, enter any comments about the session in the Comments section (generally, this is used to indicate the reason for denying credit). Participants will see anything you enter in the Comments section for their sign-up, and these comments will be included in the email sent to participants when a credit grant/revocation occurs, if notification emails are enabled on your system.
Click on the Update Sign-Ups button at the bottom of the list of sign-ups to save your changes. Credit will be granted or a penalty assessed as necessary. The participant(s) will be emailed about this if the system is configured in such a manner.
It is not recommended to leave any sign-up for a timeslot that has occurred in the “No Action Taken” stage. This is a credit “limbo” and the system will warn you upon your next login about the offending timeslot that has not been dealt with properly. Note that if Manual Cancellation is enabled and you would like to cancel a participant’s sign-up, the sign-up must be in No Action Taken state.
Depending on how your system is configured, the system may automatically grant credit to participants for timeslots that are more than an administrator-specified number of hours old, and where the researcher has taken no action. You can always change the automatic credit grant later if it was in error. The automatic credit grant takes place once a day, usually overnight. Your administrator can let you know if such a feature is enabled on your system.
If you need to do a simple credit grant across many timeslots, see the Uncredited Timeslots section which offers such a feature.
Batch Credit Granting
In some cases, you may wish to automatically sign up and immediately credit a group of participants. This is often useful if you administered a study on an ad-hoc basis, and you want to credit participants after the fact.
To do so, go to the appropriate timeslot (you may want to create a timeslot specifically for this purpose), and click on Modify Timeslot. In the Manual Sign-Up section (if enabled), you will see a Batch Credit Grant link. Click that and you can provide the list of User IDs of users you would like to sign up and credit. Users will be signed up and credited immediately. This feature overrides any sign-up restrictions on the study, just as a normal manual sign-up does.
Figure 21 – Batch Credit Grant
Emailing Participants
If you wish to contact participants in a particular timeslot for any reason, you may click on the Contact link that will appear next to each participant’s name (or ID code) to contact an individual participant. To email the group of participants for a particular timeslot, click the Contact All Participants choice at the bottom of the Modify Timeslot page for that timeslot.
You will be taken to a page where you can fill out a message that the system will send to the selected participants. The message is auto-filled with some basic information about the study, so participants are aware of which study you are referring to. You may remove this information if desired. You may choose to receive a copy of the email that you send.
Participants will be sent a reminder email by the system about upcoming studies the day before they are scheduled to participate.
Figure 22 – Contacting Participants
In some cases, you may find it useful to contact all participants for the study, across all timeslots. This feature may be particularly useful if you are sending debriefing information when a study has concluded. To do so, go to My Studies, click Study Info., next to the desired study, and choose the Contact Participants option. You will then be able to select which group of participants to send to, and a message to send. Messages will be sent in groups of 300 (or less, depending on how your system is configured) to avoid overloading email servers.
Figure 23 – Contact Participants
Viewing Uncredited Timeslots
When you login to the system, you will receive a warning if you have any timeslots that are more than 2 days old and haven’t been dealt with. You may view a list of all timeslots that have not been dealt with by choosing the View Uncredited Timeslots option from the My Studies page. The timeslots for online studies, including those in the future, are always considered in need of a response. See the Web-Based (Online) Studies section of this documentation for more information.
If you would like to do a simple credit grant (standard credit grant, no comments), you may do so directly from this page. Select the desired sign-ups/timeslots, and then choose Grant Credits. The action may take a short time to complete, so please be patient while the credit grants are processed.
If you need to do something more complex, like mark a no-show, add comments, or do a special credit grant, you can easily click on the timeslot’s date and time, and go directly to that timeslot.
In cases where a study has timeslots linked to specific researchers, you will see the warning only for timeslots that are specifically linked to you, or to everyone in the study (i.e. not timeslots linked to someone else in the study). However, when you view uncredited timeslots, you will see all uncredited timeslots for your studies, even if someone else is linked to one of the timeslots for your study. This is done to make it easier to give your fellow researchers (for your studies) assistance in dealing with uncredited timeslots.
Figure 24 – Uncredited Timeslots
Analyzing Pretest Responses
You have the opportunity to analyze pretest responses in aggregate or as raw data. Choose the Pretest Results option from the top menu bar. You can then select which question you would like to analyze, and whether you would like to see summary data or raw data (in CSV format) for the selected question. The raw data will identify each participant only by a unique ID code, not by their name, for privacy reasons. This gives you access to all pretest data across all participants in the system.
If you would like to analyze the pretest data for just those who participated in your study, select the Download Pretest Responses after clicking on your study. See Viewing Pretest Responses in this documentation for further information.
Figure 25 – Pretest Response Analysis
Pretest Qualification Analysis
If you would like to get an idea of how many participants meet a set of requirements (for help in setting pretest restrictions on your study), use the Pretest Qualification Analysis link from the Pretest Responses page. Using this feature, you can select multiple questions (only questions that qualify for study participation restriction are listed), and then the desired responses for those questions, and you will see how many participants meet that criteria.
Regulatory Compliance Guidelines
Introduction
This software complies with all major regulations governing human subject research and privacy of data stored online. The system complies with both HIPAA and Common Rule for customers in the United States. For customers in Canada, it complies with the Personal Information Protection and Electronic Documents Act as well as the Tri-Council Statement. For customers in the European Union or in countries that follow OECD rules, it complies with OECD privacy rules and the European Union Directive of Data Protection. Your organization may or may not need to comply with the relevant regulations. Your subject pool administrator can advise you on this situation.
Even if you are not required to comply, compliance is still a good idea, as protecting sensitive data is always a good thing. Compliance in the context of this system is as simple as reading the remaining paragraphs of this section (that apply to your organization) and following the guidelines contained therein. The remaining compliance issues involving software, privacy and electronic data storage are all handled automatically by the software. You should still consult with your IRB or organization to be sure there are no additional compliance rules you must follow outside of use of this software (the handling of the data you collect during your study would be one example).
Some regulations (particularly the US HIPAA regulations) are focused primarily on health data. You may think the system does not store confidential health data (in HIPAA terms, it is called PHI — Protected Health Information), but depending on how your organization uses the software, there may very well be confidential data in the system. Consider the case of a study that requires that a participant come from a family that has a history of mental illness. Merely knowing who signed up for that study is confidential because that type of information should not be revealed to the public. It may turn out that your studies are not of such a nature, but even more benign situations, like a study that requires that participants be regular contact lens wearers, can be construed as confidential information. Organizations typically err on the side of caution given the criminal and civil penalties for violation of these types of regulations.
Data Handling and Security Guidelines
In your role, you have access to your studies and you can see who has signed up for those studies. You also have access to pretest responses. Because of these privileges, you need to follow these simple guidelines:
- Secure Your Account. Use a password that is difficult to guess. The most secure passwords contain a combination of letters and numbers, do not spell a real word, and are at least 8 characters long.
- Secure Your Work Area. If you are logged into the system and you leave your computer, you should logout of the system or use a password lock on your computer.
- Handle Paper Documents Carefully. Any printouts from the system should be kept secure. Store them in a locked drawer or cabinet. Documents you decide to discard should be shredded.